GlaxoSmithKline PLC and partner Vir Biotechnology Inc. are straining to meet soaring demand for their Covid-19 antibody treatment after the highly mutated Omicron variant knocked out the two competing products.
Demand has jumped in recent weeks for the treatment, called sotrovimab, because it is the only antibody drug authorized in the U.S. for the newly infected that has been found to work against Omicron.
Glaxo and Vir, which were using one manufacturing plant, say they have raced to add another and taken other steps to roughly double the number of doses they can deliver to the U.S. in the first quarter to 600,000.
The federal government purchases doses, which it distributes to state health departments based on need. Last week, the Biden administration agreed to pay $945.1 million for the 600,000 doses.
The companies expect deliveries to start in February, which doctors say should help to ease the U.S. shortages, though supplies still won’t be enough.
“We were on the phone with the U.S. government immediately, sharing the data, discussing what was possible from a supply perspective,” said Bart Murray, who leads Glaxo’s Covid-19 business in the U.S.
Other countries are also seeking to lock up more sotrovimab doses. Australia said early this month it had reached a deal for about 45,000 doses, nearly doubling its supply. Around the same time, Canada secured 20,000 doses, adding to an earlier order for 10,000.
The scramble to expand manufacturing underscores the challenges that health authorities and drugmakers face staying ahead of an evolving virus as they roll out drugs and vaccines.
Antibody drugs are designed to mimic a part of the body’s natural immune response to Covid-19. Since first going into use in November 2020, the therapies have played an important role in pandemic treatment.
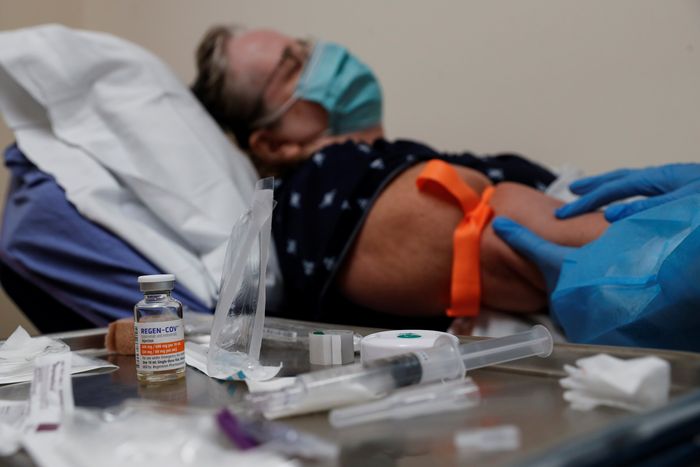
Doctors have been giving the drugs, which are administered by IV infusion, to people who are at high risk of severe disease shortly after infection, to cut the chances they will need to be hospitalized.

Before Omicron, the U.S. relied more heavily on the Regeneron and Lilly products.
Photo: Shannon Stapleton/Reuters
Three of the drugs—the other two made by Regeneron Pharmaceuticals Inc. and Eli Lilly & Co.—were authorized in the U.S. for use in people soon after infection at high risk of severe disease. A fourth, made by AstraZeneca PLC, has been authorized for preventive use in people with weakened immune systems, more akin to a vaccine.
Before Omicron, the U.S. relied more heavily on the Regeneron and Lilly products.
In the three months to Dec. 12, the federal government distributed 1.1 million courses of Regeneron’s and 582,072 courses of Lilly’s cocktail, compared with 179,880 courses of sotrovimab, according to data from the U.S. Department of Health and Human Services.
That left a huge supply hole for Glaxo and Vir to fill when Omicron emerged, because the new variant can evade the Regeneron and Lilly drugs.
“For the world, it’d be better if they all kept activity. We know we don’t have enough, and we can’t supply the need,” said Vir Chief Executive George Scangos.

As Omicron cases rise, makers of monoclonal antibody drugs have worked to diversify production in an effort to increase output.
Photo: Star Tribune/Zuma Press
Two newly authorized Covid-19 pills, from Pfizer Inc. and from Merck & Co. and partner Ridgeback Biotherapeutics LP, also have been found to work against Omicron, but they are in short supply as the companies increase production.
In December, the federal government withheld doses of sotrovimab—some weeks distributing none at all—in order to maximize supply for when Omicron overtook Delta to become the dominant strain, which happened just before Christmas.
“While we’re doing everything we can to treat as many patients as possible, it’s so overwhelming, the numbers right now,” said Amanda Peppercorn, who leads Covid-19 antibody development at Glaxo.
The National Institutes of Health updated their Covid-19 treatment guidelines on Wednesday to advise against the use of the Regeneron and Lilly treatments now that Omicron is dominant across the U.S.
In Texas, where case numbers are nearly quadruple the levels during the Delta peak, demand is outstripping supply by around 10 times.

Hospitals nationwide have been forced to ration doses of the sotrovimab monoclonal antibody treatment for their most at-risk patients.
Photo: Hannah Beier/Reuters
The Texas Department of State Health Services received 3,648 courses of sotrovimab for this week, having received requests for more than 35,000 courses from providers last week, according to a spokeswoman.
That shortage is leading hospitals to ration doses of sotrovimab for the most at-risk patients.
Valleywise Health, which runs several hospitals and clinics around Phoenix, has narrowed the eligibility for sotrovimab from all adults with health conditions that put them at increased risk of severe Covid-19 to just those 65 years and older, according to Michael White, its chief clinical officer.
“We’re always hesitant to try to prioritize among patients because we know from the studies that many people could benefit from this,” said Roy Gulick, chief of infectious disease at NewYork-Presbyterian Weill Cornell Medical Center, which is also giving priority to the most at-risk patients. “It has put providers in somewhat of an uncomfortable position.”
A single plant, run by Shanghai-based WuXi Biologics, had been responsible for global production of sotrovimab.
After Omicron hit, Glaxo and Vir said they took several steps to increase output. They accelerated efforts to add another factory, run by Samsung Biologics, to increase production. The Food and Drug Administration, which typically takes several months to approve a new plant for drugmaking, gave the companies a green light in December.
Glaxo also rejiggered supply lines at its plant in Parma, Italy, which processes the active ingredient produced by the Shanghai plant into the finished product.
“We made sure any and all available medicine that could be filled and finished for the U.S. in the time frame we needed was done,” said Mr. Murray.
Glaxo and Vir expect their global supply to continue to increase, but not all at once. Making complex drugs like sotrovimab takes time to scale up, so while the Samsung plant is already adding to global supply, it won’t run at full capacity for a few months.
Write to Denise Roland at [email protected]
Copyright ©2022 Dow Jones & Company, Inc. All Rights Reserved. 87990cbe856818d5eddac44c7b1cdeb8